Today we will learn about difference between gauge pressure and absolute pressure. Pressure is defined as a normal force exerted by a fluid per unit area. It is defined only in liquid or gas state. In the solid state the normal force per unit area is called stress. SI unit of pressure is Pascal. One Pascal is equal to one newton per meter square pressure. MTS unit of pressure is KG/cm2. Sometimes pressure is measure in respect of water column. Ten meter water column (mmwcl) is equal to one KG/cm2 pressure.
These all are small unit of pressure. The atmospheric or large quantity of pressure is measured in bars where one bar is equal to 100000 Pa pressure. One bar is also defined as 1.019 Kg/cm2 pressure. The standard value of atmospheric pressure is one bar.
According to the measurement pressure can be divided into two categories. The first one is known as gauge pressure and other one is absolute pressure. We will learn the difference between them in detail.
Difference between Gauge Pressure and Absolute Pressure:
Absolute Pressure:
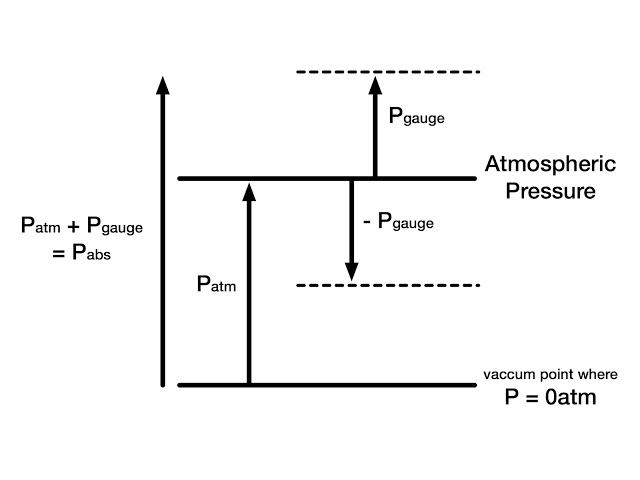
The actual pressure at a given position is called the absolute pressure and it is measured relative to absolute vacuum. One concept remembers in mind that to measure any quantity we required a base line with respect we are going to measure it.
To learn this concept supposes we need to measure distance of Bombay. Distance can be measured in meter. Can we measure distance of Bombay by this input? Obviously your answer is no because we need a reference from which we want to measure distance. Now suppose we need to measure distance of Bombay from Delhi. Now we are able to measure this distance in some meters or kilometers.
Similarly pressure cannot be measured without a reference. When we take vacuum or no pressure condition as reference the measured pressure is called absolute pressure.
Gauge Pressure:
When we take atmospheric pressure as reference to measure pressure of any system, the measured pressure is known as gauge pressure. Most of pressure devices work in atmospheric condition always measure gauge pressure. We can convert this gauge pressure in absolute pressure by adding atmospheric pressure in gauge pressure.
P (absolute) = P
(Gauge) + P (Atmospheric)
(Gauge) + P (Atmospheric)
Most of gauge read zero in atmosphere but there is some atmospheric pressure. They read atmospheric pressure as absolute zero pressure. Pressure below atmospheric pressure is called vacuum pressure and is measured by vacuum gauges that indicate the difference between the atmospheric pressure and absolute pressure.
P (vacuum) = P (Atmospheric) – P (Absolute)
This is all about gauge pressure and absolute pressure. If you have any query regarding this article, ask by commenting. If you like this article, don’t forget to share it on social networks. Subscribe our website for more informative articles. Thanks for reading it.